Our Services.
Oral Solid Dose
-
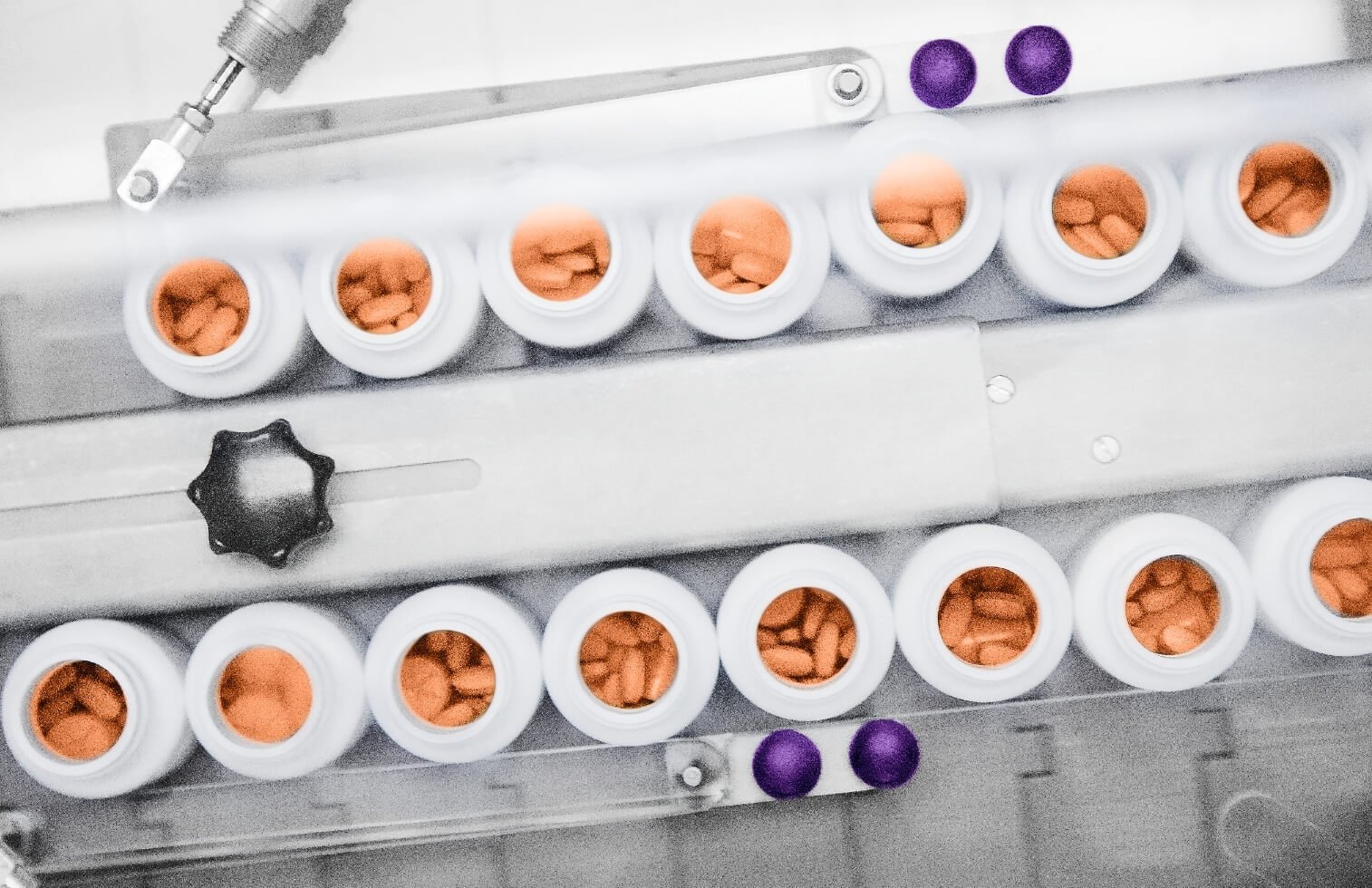
We offer clinical and commercial-scale manufacturing of OSD drug product manufacturing, including potent compounds and targeted release formulations, from facilities with a long history of serving global pharma supply chain
In line with our commitment to invest in industry best practices, Avara has expanded our OSD manufacturing capabilities to include DEA licensing for Schedule II to V controlled drugs, hot melt technology, tri-layer tablets, increased encapsulation capacity, and other custom tablet and capsule drug product manufacturing.
-
-
Oral Solid Dose (OSD) pharmaceutical manufacturing has long been a core capability of Avara, and we continue to invest in specialized equipment and flexible capacity to support projects across a range of product types and classifications.
Sterile Fill Finish
-
We offer liquid and lyophilized fill-finish of aseptic injectable products from our facility in Liscate, Italy. Our experienced teams have the flexibility to support both your clinical and commercial aseptic drug product manufacturing needs, including terminal sterilization and containment capabilities for potent compounds.
Drawing on our extensive experience and strong regulatory track record in these complex and highly scrutinized processes, we are able to safely and efficiently advance your sterile drug product.
-
-
At Avara, we are committed to meeting your aseptic liquid filling and sterile pharmaceutical manufacturing needs and priorities across every stage of your sterile fill-finish project.
Integrated Packaging
-
Our packaging capabilities include primary and secondary packaging services, automated inspection, specialty pharmaceutical packaging lines and serialization to international requirements.
-
-
Avara offers specialty pharmaceutical contract packaging solutions for your OSD and sterile drug products, with each of our global sites providing integrated packaging for the dosage forms they manufacture.